 Exam 2A, Spring 2002
Exam 2A, Spring 2002 Exam 2A, Spring 2002
Exam 2A, Spring 20021. Complete and balance the following acid/base reactions. Label the acid, base, conjugate acid, and conjugate base for each reaction.
a. aqueous nitric acid plus solid calcium hydroxide
b. aqueous perchloric acid plus water
c. HCl(aq) + CN–(aq)
d. HNO2(aq) + H2O(l)
a. Ca(OH)2(s) + 2 HNO3(aq) → 2 H2O(l) + Ca2+(aq) + 2 NO3–(aq)
Acid: HNO3
Base: Ca(OH)2
Conjugate Acid: H2O
Conjugate Base: NO3–
b. HClO4(aq) + H2O(l) → H3O+(aq) + ClO4–(aq)
Acid: HClO4
Base: H2O
Conjugate Acid: H3O+
Conjugate Base: ClO4–
c. HCl(aq) + CN–(aq) → HCN(aq) + Cl–(aq)
Acid: HCl
Base: CN–
Conjugate Acid: HCN
Conjugate Base: Cl–
d. HNO2(aq) + H2O(l) → ← H3O+(aq) + NO2–(aq)
Acid: HNO2
Base: H2O
Conjugate Acid: H3O+
Conjugate Base: NO2–
2. Write the chemical reaction needed to determine the pH and give the pH for the following solutions.
a. 0.0010 M hydroiodic acid.
b. 0.0010 M strontium hydroxide
a. 0.0010 M hydroiodic acid.
HI(aq) + H2O(l) → H3O+(aq) + I–(aq)
[H3O+] = [HI] = 0.0010 M
pH = –log[H3O+] = –log[0.0010] = 3.00
b. 0.0010 M strontium hydroxide
Sr(OH)2(aq) → Sr2+(aq) + 2 OH–(aq)
[OH–] = 2×[Sr(OH)2] = 2×[0.0010] = 2.0×10–3 M
pOH = –log[OH–] = –log[2.0×10–3] = 2.70
pH = 14.00 – pOH = 14.00 – 2.70 = 11.30
3. Indicate the stronger acid in each pair. Explain your reasoning in 5 words or less. For the stronger acid, write out the balanced reaction of the acid with water.
a. CF3COOH vs. CH3COOH
b. H4SiO4 vs. H3PO4
c. HCrO4– vs. HMnO4
a. CF3COOH vs. CH3COOH
CF3COOH because of the F electronegativity
CF3COOH(aq) + H2O(l) → ← H3O+(aq) + CF3COO–(aq)
b. H4SiO4 vs. H3PO4
H3PO4 because of more oxo-type O atoms
H3PO4(aq) + H2O(l) → ← H3O+(aq) + H2PO4–(aq)
c. HCrO4– vs. HMnO4
HMnO4 because of charge
HMnO4(aq) + H2O(l) → ← H3O+(aq) + MnO4–(aq)
4. Complete the following reactions and indicate which direction equilibrium will favor:
a. HClO2(aq) + BrO2–(aq)
b. NH3(aq) + HF(aq)
a. HClO2(aq) + BrO2–(aq) → ← HBrO2(aq) + ClO2–(aq)
Equilibrium favors the side with the weaker acid, which is HBrO2 (electronegativity), so the products side is favored.
b. NH3(aq) + HF(aq) → ← NH4+(aq) + F–(aq)
Equilibrium favors the side with the weaker acid, which is HF (charge), so the reactants side is favored.
5. A 0.0010 M solution of hypoiodous acid (HIO, Ka = 2.3×10–11) was prepared. Find the pH.
HIO(aq) + H2O(l) → ← H3O+(aq) + IO–(aq)
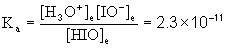
Initial 0.0010 0 0
Change –x +x +x
Equilibrium 0.0010 – x x x
Approximate? 0.0010/2.3×10–11 = 4.3×107: Yes
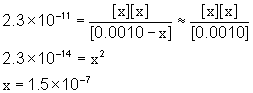
[H3O+]e = 1.5×10–7 M
pH = –log[H3O+] = –log[1.5×10–7] = 6.82