 Exam 1B, Spring 2006
Exam 1B, Spring 2006 Exam 1B, Spring 2006
Exam 1B, Spring 20061. Consider the reaction between hydrogen carbonate ion and hydrogen phosphate ion that gives dihydrogen phosphate ion and carbonate ion as products.
a. Write the balanced reaction.
b. Assuming that this is an elementary reaction, write the rate law.
HPO42–(aq) + HCO3–(aq) → H2PO4–(aq) + CO32–(aq)
b. Rate = –k[HPO42–][HCO3–]
2. Briefly (15 words or less) distinguish between a rate law and a rate constant.
Rate law relates the rate of reaction and concentrations. Rate constant = probability factor in the rate law.
3. For the reaction: A(g) + B(g) → Products; the initial rate is 0.056 mole·L–1s–1. The reaction was allowed to continue and the rate was measured again 10 minutes later. The rate found in the second measurement was 0.057 mole·L–1s–1. What is the order of the reaction?
Since the rate is the same at two different times during the course of the reaction, this must be a zero order reaction.
4. A reaction, A(g) → Products, was studied. In the first experiment the initial concentration was [A]o = 1.200 M; after 10.0 s the concentration the reactant was found to be [A] = 1.100 M. In the second experiment the initial concentration was [A]o = 1.600 M and after 100.0 s [A] = 0.600 M.
a. What is the initial rate for the first experiment?
b. What is the initial rate for the second experiment?
c. What is the rate law?
d. What is the value of the rate constant?
a. Rate = (1.100 – 1.200)/(10.0 – 0) = –0.0100 Ms–1
b. Rate = (0.600 – 1.600)/(100.0 – 0) = –0.0100 Ms–1
c. Since the initial rates are the same for two different initial concentrations, this must be a zero order reaction. Thus,
Rate = –k
d. k = –Rate = 0.0100 Ms–1
5. Consider the reaction:
S2O82–(aq) + 3 I–(aq) → 2 SO42–(aq) + I3–(aq)
The following data was measured:
Experiment [S2O82–] (M) [I–] (M) Initial Rate (M min–1)
1 0.10 0.10 7.0×10–5
2 0.10 0.20 1.4×10–4
3 0.30 0.10 2.1×10–4
a. What is the rate law for the reaction?
b. What is the value of the rate constant for the reaction?
a. Since the rate doubles when the iodide ion concentration doubles and the persulfation concentration is held constant (experiments 1 & 2), this must be first order in iodide ion. Similarly, when the concentration of persulfate ion triples and the iodide ion concentration is held constant (experiments 1 & 3), the initial rate triples so the reaction is also first order in persulfate ion. Thus, the rate law is:
Rate = –k[S2O82–][I–]
b. Using experiment 1, k = 7.0×10–5/(0.10)(0.10) = 7.0×10–3 M–1s–1 (The other experiments give identical values.)
6. The following mechanism was proposed for a reaction:
OCl–(aq) + H2O(l)
HOCl(aq) + OH–(aq) fast
I–(aq) + HOCl(aq)
HOI(aq) + Cl–(aq) slow
HOI(aq) + OH–(aq)
H2O(l) + OI–(aq) fast
a. Write the net reaction.
b. Predict the rate law based on this mechanism.
a. Add all three reactions together:
OCl–(aq) + H2O(l) + I–(aq) + HOCl(aq) + HOI(aq) + OH–(aq) → HOCl(aq) + OH–(aq) + HOI(aq) + Cl–(aq) + H2O(l) + OI–(aq)
Eliminate common species (HOCl, OH–, HOI, and H2O):
OCl–(aq) + I–(aq) → Cl–(aq) + OI–(aq)
b. The slow step is rate-determining, so the rate law must derive from:
Rate = –k2[I–][HOCl]
HOCl is an intermediate, created by step 1, so must be eliminated using the fast reaction in the first step:
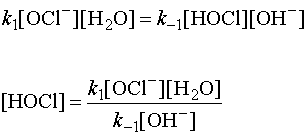
Substituting into the rate law from the rate-determining step and doing some algebra:
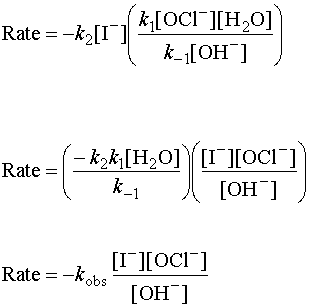
Water is grouped with the constants because the concentration of a solvent does not change much during the course of a reaction. Hydroxide ion is an inhibitor in this reaction.
7. The following data was found for a first order reaction:
time (s) [reactant] (M)
0 1.00
1 0.79
2 0.63
3 0.50
4 0.40
What is the half–life of the reaction?
By the definition of half–life (the time required to reach half of the initial concentration), t½ = 3 s
8. Consider the three reaction containers shown below:



A B C
The rate law is given by Rate = k[o]. Which reaction container will react fastest? Briefly explain your reasoning.
Since the rate law is first order in open circles, the rate will be highest when the concentration of open circles is highest. Container B has the most open circles in the given volume, so this reaction must be fastest.
9. Catalysis increases the rate of a reaction by lowering the activation energy. Briefly explain how this occurs.
The catalyst reacts with one or more reactants to form a new activated complex that has a lower activation energy than the uncatalyzed reaction. A lower energy barrier leads to a faster reaction.