 Exam 1B, Spring 2003
Exam 1B, Spring 2003 Exam 1B, Spring 2003
Exam 1B, Spring 20031. In aqueous solution, hydrogen phosphate ion reacts with hydrogen carbonate ion to give carbonate ion and dihydrogen phosphate ion. 1.0 mole of each reactant was placed in a 1.0 L vessel and allowed to react; after 10.0 s, 0.9 mole of carbonate ion was found to be remaining in the reaction flask.
a) Write the balanced chemical reaction.
b) What is the rate of gain of carbonate ions?
c) What is the rate of loss of hydrogen phosphate ions?
a) HCO3–(aq) + HPO42–(aq) → H2PO4–(aq) + CO32–(aq)
b) 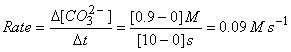
c) Rate = –0.09 M s–1 since the stoichiometry is 1:1
2. Dinitrogen pentoxide gas decomposes into nitrogen dioxide gas and dioxygen gas at 335 K. The time evolution of the loss of dinitrogen pentoxide was measured and the data plotted as shown below.
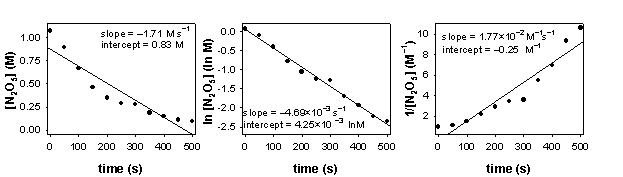
a) Write the balanced chemical reaction.
b) Determine the order of the reaction. Explain how you found the order.
c) Find the rate constant for the reaction at 335 K. Give the units of the rate constant.
a) 2 N2O5(g) → 4 NO2(g) + O2(g)
b) 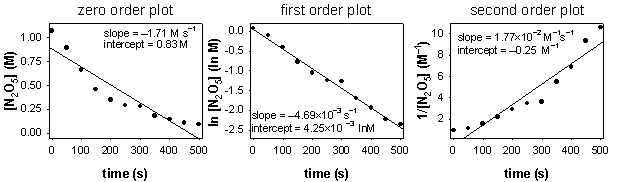
The plot of ln[N2O5] vs. t is linear, indicative of a first order reaction.
c) The slope of the first order plot gives the rate constant, k = –slope = 4.69×10–3 s–1.
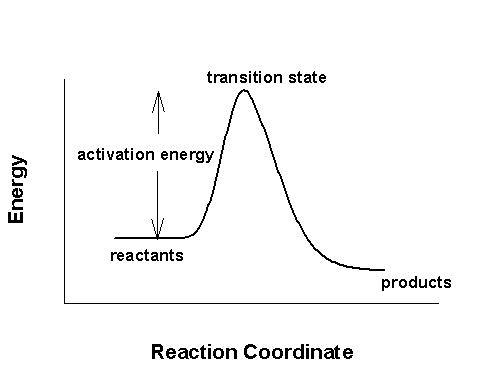
3. Draw the transition state diagram for an exothermic reaction. Label the axes, reactants, products, activated complex, and the activation energy.
4. Write the mass action expressions for Kc for the following reactions:
a) N2O(g) + NO2(g) → ← N2(g) + O2(g)
b) CH4(g) → ← C(s) + H2(g)
c) H2CO3(aq) → ← CO2(g) + H2O(l)
Balance: 2 N2O(g) + 2 NO2(g) → ← 3 N2(g) + 3 O2(g)
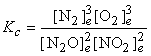
b) Balance: CH4(g) → ← C(s) + 2 H2(g)
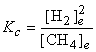
c) Already balanced:
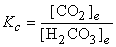
5. For the reaction N2(g) + O2(g) + Cl2(g) → ← NOCl(g), a mixture containing each reactant at a partial pressure of 2.0 atm was allowed to react. When equilibrium was established, the partial pressure of nitrogen was found to be 1.0 atm. Find Kp for the reaction.
Balance: N2(g) + O2(g) + Cl2(g) → ← 2 NOCl(g
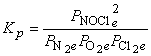
Initial: 2.0 2.0 2.0 0
Change: –x –x –x +2x
Equilibrium: 2.0 – x 2.0 – x 2.0 – x 2x
At equilibrium, 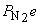 = 1.0 atm = 2.0 – x, so x = 1.0 atm
=
= 1.0 atm = 2.0 – x, so x = 1.0 atm
= 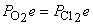 and
and 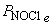 = 2x = 2.0 atm
= 2x = 2.0 atm
![]()
6. The following reaction was allowed to come to equilibrium.
CO(g) + H2(g) → ← C(s) + H2O(g) ΔH° = –131 kJ/mole
Which way does the reaction shift if:
a) carbon monoxide is added to the reaction?
b) the temperature is raised?
c) the pressure is increased by reducing the volume of the reaction vessel?
d) a small amount of graphite is removed from the reaction vessel?
a) Right, towards products to remove some of the added CO.
b) Left, towards reactants to remove some of the added heat.
c) Right, towards products, which has fewer moles of gases so can reduce some of the increased pressure.
d) No shift; since the graphite is a solid its concentration does not change so does not affect the equilibrium position.
7. Consider the following mechanism for some hypothetical reaction:
A(g) + B(g) → ← C(g) k1 = 0.1 M–1s–1, Kp = 10
C(g) + B(g) → ← D(g) k2 = 100 M–1s–1, Kp = 1
a) Write the net reaction.
b) Write the rate law for the net reaction.
c) Find the numerical value for Kp for the net reaction.
a) A(g) + 2 B(g) → ← D(g)
b) The first step is rate-limiting because it has the smaller rate constant, so the rate law is:
Rate = k1[A][B]
c) The equilibrium constant for the net reaction is found from the product of the equilibrium constants for each step:
Kp = (10)(1) = 10