 Exam 1B, Spring 1999
Exam 1B, Spring 1999 Exam 1B, Spring 1999
Exam 1B, Spring 19991. Briefly, distinguish between a reaction mechanism and a reaction intermediate.
A reaction mechanism is a list of all the elementary reactions that occur during an overall reaction. A reaction intermediate is a species that is short-lived that may be created as one step in a reaction mechanism.
2. How does a reaction quotient differ from an equilibrium constant? Be brief.
A reaction quotient is evaluated at any set of conditions. An equilibrium constant can only be evaluated at equilibrium conditions.
3. For the reaction For the reaction H2O2(aq) + 2 Fe2+(aq) + 2 H+(aq) → 2 Fe3+(aq) + 2 H2O(l) the rate law was found to be Rate = k[H2O2][Fe2+]. What is the order of reaction in each reactant?
First order in H2O2; first order in Fe2+, zero order in H+. These are based on the rate law, not the reaction stoichiometry.
4. Write the mass action expression for Kp for the reaction: H2(g) + I2(g) →← HI(g)
The equation needs to be balanced: H2(g) + I2(g)→ ← 2 HI(g)
Then the mass action expression for Kp becomes:
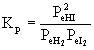
5. Write the mass action expression for Kc for the reaction: BaCO3(s) →← BaO(s) + CO2(g)
Pure solids do not contribute, so Kc = [CO2]e
6. The following data was collected for the reaction C6H5N2Cl(aq) → C6H5Cl(aq) + N2(g)
t (min)[C6H5N2Cl] (M)
00.0710
60.0475
120.0315
180.0207
240.0144
300.0096
Make a first order plot of the data. Is the reaction first order? Why or why not?
Make a first order plot of the data. Is the reaction first order? Why or why not?
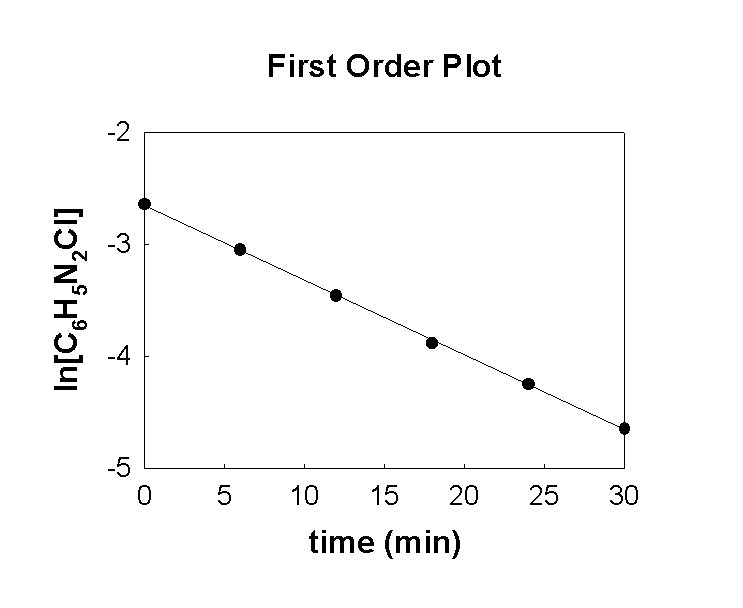
The plot is linear with very little error. The reaction is almost certainly first order.
7. A rate law for a reaction was found to be Rate = k[NO]2[Cl2] The following mechanism was proposed:
NO(g) + Cl2(g) → NOCl(g) + Cl(g) Slow
NO(g) + Cl(g) → NOCl(g) Fast
a. Write the overall reaction.
b. Is this a plausible mechanism? Why or why not?
a. Write the overall reaction.
Add the two reactions together:
2 NO(g) + Cl2(g) → 2 NOCl(g)
b. Is this a plausible mechanism? Why or why not?
No, the rate-determining step is the first one and the rate law for this elementary reaction is first order in NO, in contrast to the experiment, which is second order in NO.
8. Consider the exothermic reaction of nitrogen with hydrogen to give ammonia at equilibrium. Which way will the reaction shift if
a. Hydrogen is added to the system?
b. The pressure is decreased?
c. The temperature is decreased?
d. Fe is added as a catalyst?
a. Hydrogen is added to the system?
Shifts right, (→) towards products, to remove the added hydrogen.
b. The pressure is decreased?
Shifts left, (←) towards reactants, to increase the number of moles of gases.
c. The temperature is decreased?
Shifts right, (→) towards products, to replace the heat removed.
d. Fe is added as a catalyst?
No change - catalysts accelerate reaction rates but do not change the equilibrium composition.
9. For some reaction, the following rate data was measured:
T (°C)k (s–1)
4504.71×10–3
5509.39×10–1
a. What is the activation energy in kJ/mol?
b. What is the half-life at each temperature?
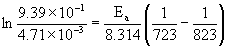
solving gives Ea = 262 kJ/mol
b. What is the half-life at each temperature?
The units of the rate constant indicate that the reaction is first order so t½ = 0.693/k
T1 = 450 °C t½ = 0.693/4.71×10–3 = 147 s
T2 = 550 °C t½ = 0.693/9.39×10–1 = 0.738 s