 Exam 1A, Spring 2004
Exam 1A, Spring 2004 Exam 1A, Spring 2004
Exam 1A, Spring 20041. Nomenclature:
a. Give the name for HPO42–
b. Give the molecular formula for the nitrate ion.
2. The following reaction has a rate constant k = 1.0×10–10 s–1 at 25 °C.
[Co(NH3)6]2+(aq) + H2O(l) → [Co(NH3)5(H2O)]2+(aq) + NH3(aq)
a. Write the rate law for the reaction.
b. What is the initial rate of reaction if the initial concentration of [Co(NH3)6]2+ is 1.0 mM?
c. If the initial concentration of [Co(NH3)6]2+ is 1.0 M, what is the half-life of the reaction?
d. Is this an elementary reaction? Why or why not?
3. A student measured the rate constants at several temperatures for the following reaction and made the plots shown.
[Ir(Cl)(CO)(P(C6H5)3)2](benzene) + H2(g) → [Ir(H2)(Cl)(CO)(P(C6H5)3)2](benzene)
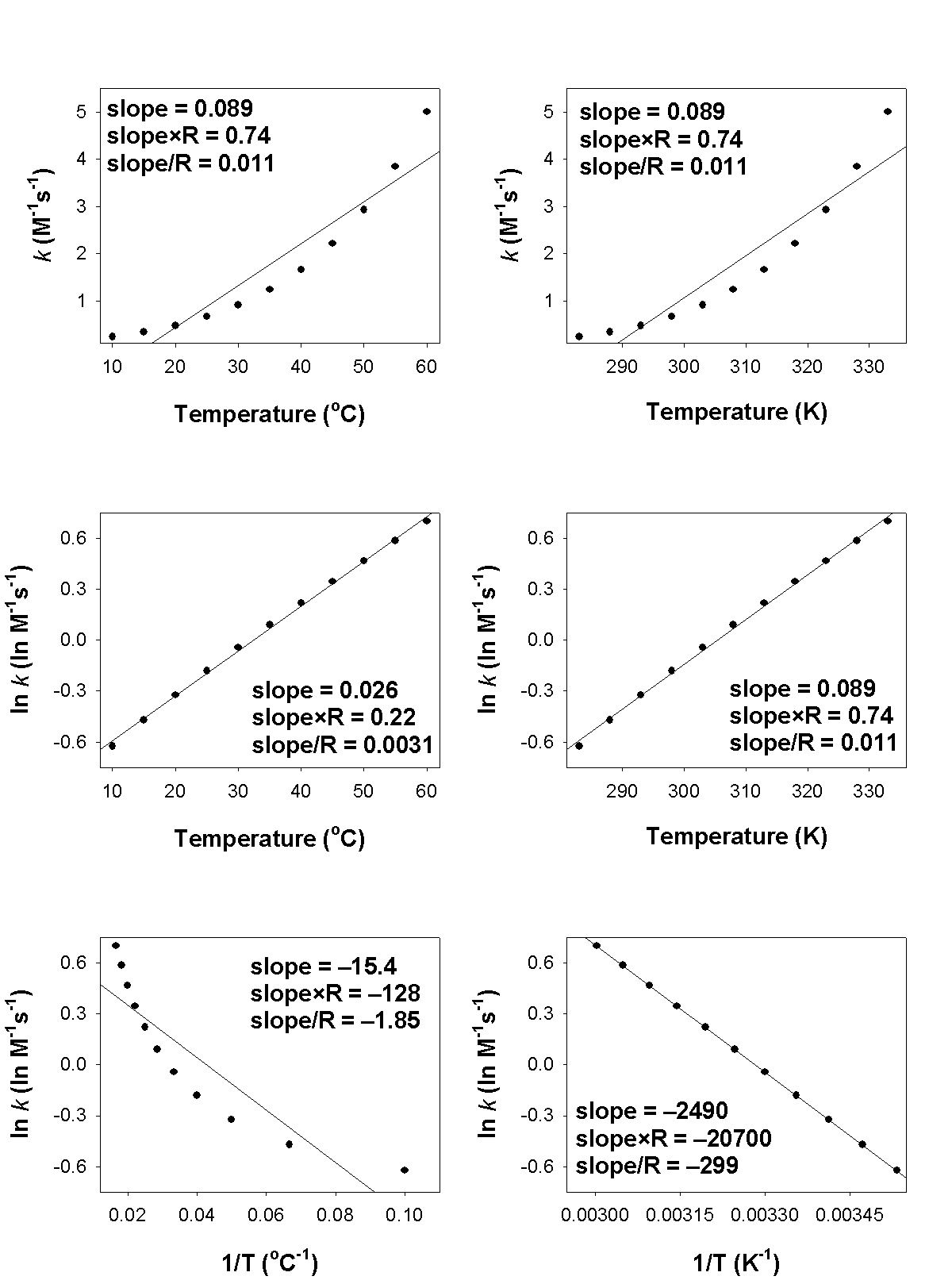
a. What is the order of reaction?
b. What is the rate constant for the reaction at 30 °C?
c. What is the activation energy in units of kJ/mol? The value of R used for the indicated calculations is 8.314 J/mol·K
4. The data listed below was collected for the following reaction:
[Cr(CO)4(P(OC6H5)3)(As(C6H5)3)] → products
Time (min)Concentration (M)
20.374
70.312
140.259
220.203
320.155
430.108
540.078
670.050
a. What is the order of reaction?
b. Estimate the half-life in units of minutes.
5. The following mechanism was proposed for a reaction:
[IrCl6]2–(aq) + [W(CN)8]4–(aq) → ← [IrCl62–---W(CN)84– ](aq)
[IrCl62–---W(CN)84– ](aq) → ← [IrCl63–---W(CN)83– ](aq)
[IrCl63–---W(CN)83– ](aq) → ← [IrCl6]3–(aq) + [W(CN)8]3–(aq)
a. Write the net reaction.
b. What is the molecularity of each reaction in the mechanism?
c.Which step is rate determining? Why?
d. Write the rate law for the net reaction.
6. Write the mass action expression for Kc for the following reactions.
a. H2(g) + I2(g) → ←HI(g)
b. CO(g) + H2(g) → ←CH3OH(g)
c. HCl(g) + NH3(g) → ←NH4Cl(s)
7. Consider the following set of reactions:
A(g) → ← B(g) Kp = 10
B(g) + C(g) → ← D(g) Kp = 100
D(g) → ← E(g) Kp = 0.1
Find the equilibrium constant for the reaction:
A(g) + E(g) → ← 2 B(g) + C(g)