 Exam 1A, Spring 2000
Exam 1A, Spring 2000 Exam 1A, Spring 2000
Exam 1A, Spring 20001. Consider the reaction: NO(g)+ H2(g) → ½N2 (g) + H2O(g)
a. The nitrogen monoxide and hydrogen concentrations are initially set to 0.0150 M. After 25 s, the concentration of nitrogen monoxide has decreased to 0.0080 M. What is the initial rate of decrease of nitrogen monoxide? Give the units.
b. Using the same conditions as in part a, what is the rate of increase of nitrogen? Give the units.
a. Rate of loss of NO is 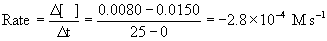
b. The rate of gain of N2 is determined by stoichiometry: –½(rate of loss of NO) = –½(–2.8×10–4) = +1.4×10–4 M s–1
2. The following data was measured for the reaction: S2O82–(aq) + 3 I–(aq) → 2 SO42–(aq) + I3– (aq)
Experiment [S2O82–] (M) [I–] (M) Initial rate (M s–1)
1 0.100 0.100 6.15×10–5
2 0.100 0.200 1.21×10–4
3 0.300 0.100 1.90×10–4
a. Find the order of reaction in each reactant.
b. Find the average rate constant. Give the units.
Rate = k[S2O82–]m[I–]n
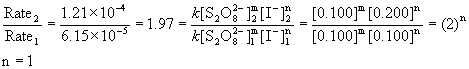
First order in iodide.
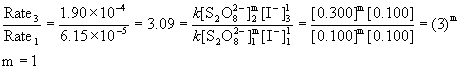
First order in persulfate
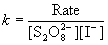
k1 = 6.15×10–5/[0.100][0.100] = 6.15×10–3M–1 s–1
k2 = 1.21×10–4/[0.100][0.200] =6.05×10–3 M–1 s–1
k3 = 1.90×10–4/[0.300][0.100] =6.33×10–3 M–1 s–1
kave = 6.18×10–3 M–1 s–1
3. For the reaction [Co(CN)5(H2O)]2–(aq) + N3–(aq) → [Co(CN)5(N3)]3–(aq) + H2O(l) the following data was measured:
T (°C) k (s–1)
20 3.9×10–8
29 8.7×10–8
37 3.6×10–7
43 9.6×10–7
49 1.2×10–6
Make an Arrhenius plot of the data (making the most effective use of the graph paper) and find the best straight line to estimate the activation energy in units of kJ/mol.
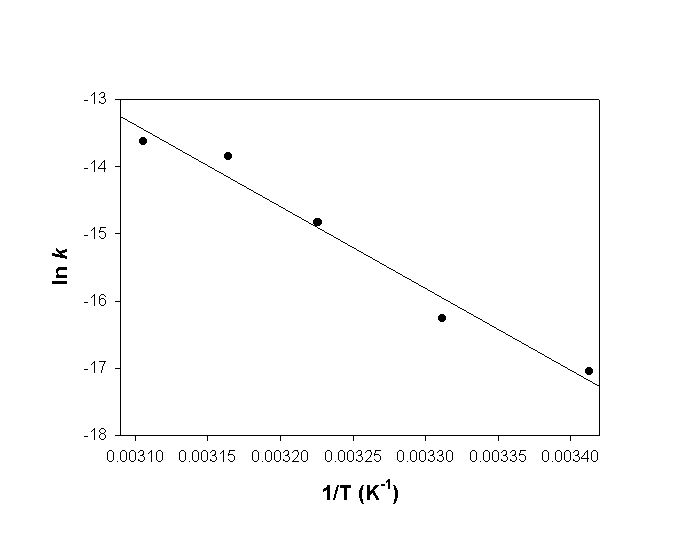
slope = –12200 = –Ea/R
so Ea = 12200(8.314) = 101000 J/mol = 101 kJ/mol
4. A student measured the half-life of a single reactant decomposition to be 1045 s when the initial concentration of reactant was 0.10 M. The student’s friend started with an initial concentration of 0.0050 M and found the half-life to be 52 s. What is the order of the reaction and what is the rate constant? Give the units of the rate constant.
The reaction cannot be first order because the half-life changes with initial concentration.
If the reaction is zero order: 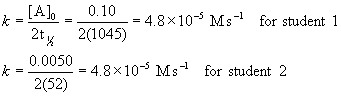
If the reaction is second order: 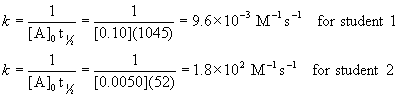
Since the rate constants must be the same for any initial concentration, the reaction must be zero order with k = 4.8×10–5 M s–1
5. Write the mass action expression for Kc for the following reactions:
a. Kr(g) + F2(g) → ← KrF2(g)
b. N2(g) + O2(g) → ← NO(g)
c. C(s) + O2(g) → ← CO(g)
a. 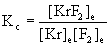
b. 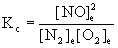
c. BALANCE FIRST: 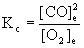
6. Find the equilibrium constant Kp for the reaction: HBr(g) →← H(g) + Br(g), given the following data (T = 1400 K):
H2(g) + Br2(g) → ← 2 HBr(g)Kp = 6.7×104
H2(g) → ← 2 H(g)Kp = 2.96×10–11
Br2(g) → ← 2 Br(g)Kp = 3.03×10–2
Use the Law of Multiple Equilibria
Sum the reactions to give the target reaction.
Multiply the equilibrium constants: Kp = Kp(1)× Kp(2)× Kp(3)
= (1.5×10–5)(2.96×10–11)(3.03×10–2) = 1.3×10–17
7. When chlorine molecules react with water vapor the products are hydrogen chloride gas and oxygen molecules, a reversible, endothermic reaction. Equilibrium is established for the reaction. Which way does the reaction shift if:
a. water is added to the reaction mixture?
b. the total pressure is increased by decreasing the volume of the reaction vessel?
c. the temperature is increased?
The balanced reaction: 2 Cl2(g) + 2 H2O(g) + heat →← 4 HCl(g) + O2(g)
a.shifts right (→) to remove added water
b. shifts left (←) to fewer moles of gases to reduce the pressure
c. shifts right (→) to remove the added heat
8. For the reaction PCl5(g) → ← PCl3(g) + Cl2(g), the initial concentration of PCl5 is 0.210 M at 250 °C where Kc= 0.038. What are the equilibrium concentrations of all components in the reaction?
PCl5(g) → ← PCl3(g) + Cl2(g)
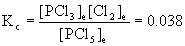
Initial 0.210 0 0
Change –x +x +x
Equilibrium 0.210–x x x
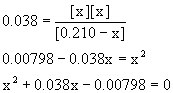
Using the quadratic equation gives
x = 0.072 or x = –0.11
only the positive value makes sense chemically, so
x = [PCl3]e = [Cl2]e = 0.072 M
[PCl5]e = 0.210 – x = 0.210 – 0.072 = 0.138 M