 Exam 1A, Spring 1999
Exam 1A, Spring 1999 Exam 1A, Spring 1999
Exam 1A, Spring 19991. Briefly, distinguish between a rate law and a rate constant.
A rate law denotes the change of concentration with time for a reaction for any time or concentration of reactants. The rate constant is a probability factor used in the rate law.
2. How does a reaction quotient differ from an equilibrium constant? Be brief.
A reaction quotient is evaluated at any set of conditions. An equilibrium constant can only be evaluated at equilibrium conditions.
3. For the reaction CHCl3(g) + Cl2(g) → CCl4(g) + HCl(g) the rate law was found to be Rate = k[CHCl3][Cl2]1/2. What is the order of reaction in each component?
First order in CHCl3; one-half order in Cl2. This is based on the rate law, not the reaction stoichiometry.
4. Write the mass action expression for Kp for the reaction: H2(g) + Br2(g) →← HBr(g)
The equation needs to be balanced: H2(g) + Br2(g) →← 2 HBr(g)
Then the mass action expression for Kp becomes:
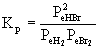
5. Write the mass action expression for Kc for the reaction: CaCO3(s) →← CaO(s) + CO2(g)
Pure solids do not contribute, so Kc = [CO2]e
6. The following data was collected for the reaction C6H5N2Cl(aq) → C6H5Cl(aq) + N2(g)
t (min)[C6H5N2Cl] (M)
00.0710
60.0475
120.0315
180.0207
240.0144
300.0096
Make a zero order plot of the data. Is the reaction zero order? Why or why not?
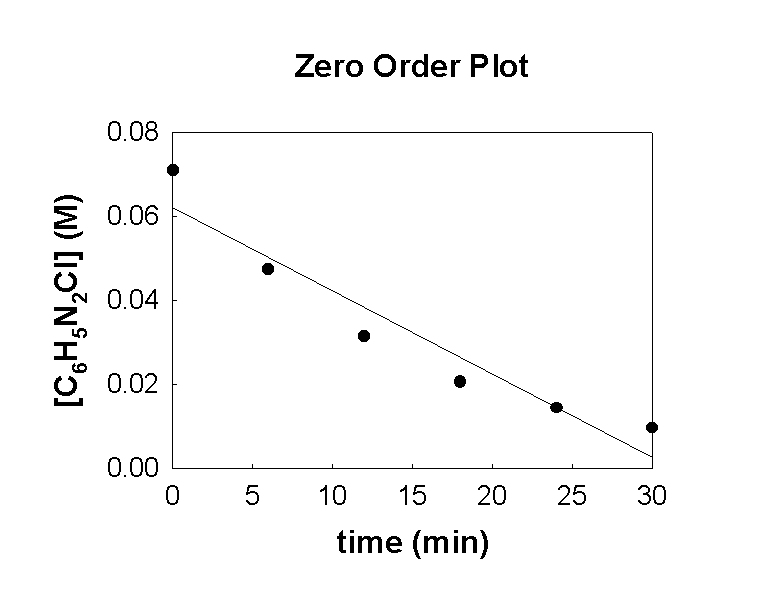
A zero order plot is [ ] vs. t. The data is clearly nonlinear so the reaction cannot be zero order.
7. A rate law for a reaction was found to be Rate = k[NO2]2. The following mechanism was proposed:
N2O4(g) + 2 CO(g) → 2 NO(g) + 2 CO2(g) Slow
2 NO2(g) → N2O4(g) Fast
a. Write the overall reaction.
b. Is this a plausible mechanism? Why or why not?
a. Write the overall reaction.
Add the two reactions together to give:
2 NO2(g) + 2 CO(g) → 2 NO(g) + 2 CO2(g)
reduce the coefficients all by a factor of 2:
NO2(g) + CO(g) → NO(g) + CO2(g)
b. Is this a plausible mechanism? Why or why not?
No. The rate law for the slow step does not match the experimental rate law.
8. Consider the exothermic reaction of nitrogen with hydrogen to give ammonia at equilibrium. Which way will the reaction shift if
a. Ammonia is added to the system?
b. The pressure is increased?
c. The temperature is increased?
d. Fe is added as a catalyst?
a. Ammonia is added to the system?
Shifts left (←) towards reactants, to remove the added ammonia.
b. The pressure is increased?
Shifts right (→) towards products, to a lower number of moles of gases.
c. The temperature is increased?
Shifts left, (←) towards reactants, to remove the added heat.
d. Fe is added as a catalyst?
No change - catalysts accelerate reaction rates but do not change the equilibrium composition.
9. For some reaction, the following rate data was measured:
T (°C)k (s–1)
2502.72×10–10
3504.31×10–6
a. What is the activation energy in kJ/mol?
b. What is the half-life at each temperature?
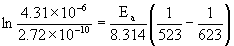
solving gives Ea = 262 kJ/mol
b. What is the half-life at each temperature?
The units of the rate constant indicate that the reaction is first order so t1/2 = 0.693/k
T1 = 250 °C t½ = 0.693/2.72×10–10 = 2.55×109 s (about 81 years!)
T2 = 350 °C t½ = 0.693/4.31×10–6 = 1.61×105 s (about 45 hours)