
The solubility product constant of calcium hydroxide was measured at several temperatures, as given below. Find ΔH° and ΔS° using a van't Hoff plot.
T (°C)Ksp T (K) 1/T (K–1) ln Ksp
105.5×10–6 283 0.00353 –12.11
204.8×10–6 293 0.00341 –12.25
303.2×10–6 303 0.00330 –12.65
402.7×10–6 313 0.00319 –12.82
502.5×10–6 323 0.00310 –12.90
601.9×10–6 333 0.00300 –13.17
701.5×10–6 343 0.00292 –13.41
801.5×10–6 353 0.00283 –13.41
901.2×10–6 363 0.00275 –13.63
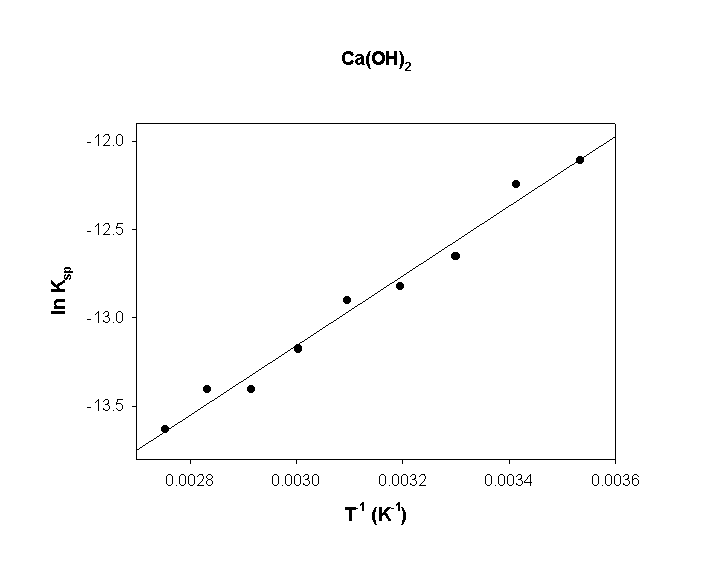
slope = 1970
intercept = –19.1