A reaction mechanism is the list of elementary reactions required to give rise to an observed reaction.
Why do we care about this?
If we know the details of a reaction, it may allow us to modify the reaction in some favorable manner.
Knowledge of the reaction mechanism also can help us account for the observed rate law for a reaction.
The second point is the one we want to focus on.
We can use reaction mechanisms to account for observed rate laws because we can write the rate laws for each elementary reaction in a mechanism by inspection. By using the rate laws for the elementary reactions appropriately, the rate law for the overall, macroscopic reaction can be determined.
1. The stoichiometry of the mechanism must match the observed stoichiometry.
2. The rate law deduced from the mechanism must match the observed rate law.
Even with these restrictions, generating a mechanism is difficult and relies on a lot of guesswork. Our goal here is to be able to determine if a proposed mechanism is consistent with experimental data, at least in simple cases.
Often the rate of an overall reaction is determined by a single elementary reaction – called the rate–determining step.
For an elementary reaction to be a rate–determining step it must have a slower reaction rate than any other reaction in the mechanism.
Sometimes the molecularity of a reaction can be used to guess if an elementary reaction is a rate–determining step.
Molecularity is the count of reacting particles in an elementary reaction:
Number of Particles Molecularity
1 Unimolecular
2 Bimolecular
3 Termolecular
Termolecular reaction are almost always rate limiting because it is a low probability event to get three particles to hit in the same location at the same time.
Reaction mechanisms can also create molecules that are not present in either the reactants or products. These are called intermediates.
Consider the reaction:
H2(g) + I2(g) → 2 HI(g)
The observed rate law is first order in each reactant.
The proposed mechanism for this reaction is:
I2(g) → 2 I(g) [1] k1 (fast)
2 I(g) → I2(g) [2] k–1 (fast)
H2(g) + 2 I(g) → 2 HI(g) [3] k2 (slow) (Why is this the slow step?)
The first two reactions are better written as a single reaction:
I2(g) → k1 ← k–1 2 I(g)
The third reaction is
H2(g) + 2 I(g) → k2 2 HI(g)
Now, when the reactions are summed, the result is:
H2(g) + 2 I(g) + I2(g) → 2 HI(g) + 2 I(g)
(the colors show the origin of each term)
The 2 I(g) quantities cancel (chemical equations can be treated like algebraic equations, like terms on each side of the arrow subtract out)
to give the net equation
H2(g) + I2(g) → 2 HI(g)
which is the observed reaction.
For elementary reactions, the order of reaction for a reactant equals its stoichiometric coefficient (Remember: this is true only for elementary reactions!)
Thus, the rate laws for each step in the proposed mechanism can be written down by inspection:
Step 1 Rate = k1[I2]
Step 2 Rate = k–1[I]2
Step 3 Rate = k2[H2][I]2
The slow step is rate limiting, so if this is an acceptable mechanism, then the rate law should match the experimental rate law.
It doesn't! The overall order is wrong (3rd vs. an observed overall order of 2) and there is an intermediate as part of the rate law. Neither of these observations is acceptable.
Can we fix this?
If step 1 and step 2 are both fast, then compared to the rate–determining step they are the same:
Rate(1) = Rate(2)
k1[I2] = k–1[I]2
or
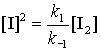
If we substitute this into the rate law from step 3
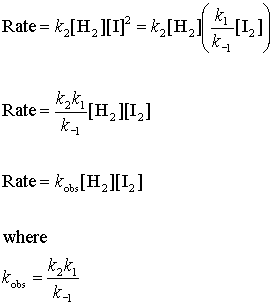
Now the observed rate law matches the rate law predicted by the mechanism, so this is a plausible mechanism.
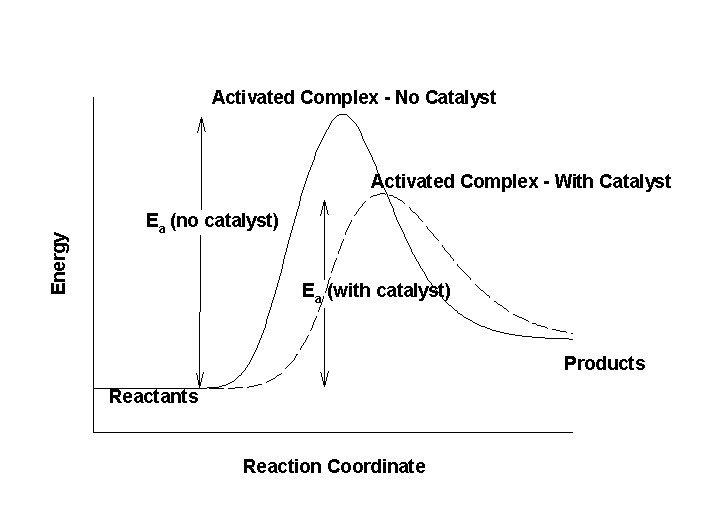
Catalysts accelerate reactions by becoming part of the transition state activated complex. They can show up as part of mechanisms so the rate constant associated with the elementary reactions that the catalyst participate in become part of the observed rate constant. Structurally, the activated complex that contains a catalyst is of lower energy than without the catalyst, which has the effect of lowering the activation energy.
There are 2 categories of catalysts:
Heterogeneous catalysts: these are catalysts that are in a different phase than the reacting medium. Typically, these are solids used to catalyze a solution phase reaction.
Homogeneous catalysts: these are catalysts in the same phase as the reacting medium.