
Ionic salts dissolved in water often affect the pH of the solution. This results from hydrolysis reactions.
Hydrolysis: the reaction of a substance with water.
Both cations and anions can undergo hydrolysis, but not all ions react in this fashion.
When an ionic salt dissolves in water, both the free cation and the free anion are formed. Thus, we need to examine both ions in order to determine the acid/base character of a salt.
Typically, this is a three step process.
Write the reaction that dissociates the salt into its constituent ions.
Check the cation for hydrolysis.
Check the anion for hydrolysis.
A water-soluble salt dissociates completely into ions:
MpXq(aq) → p Mq+(aq) + q Xp–(aq)
The stoichiometry of the dissociation is important for quantitative applications but less so for qualitatitive assessments.
The charges on the ions, of course, are critical.
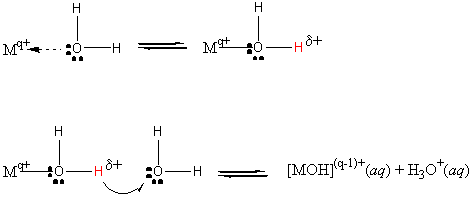
Mq+(aq) + 2 H2O(l) ? MOH(q–1)+(aq) + H3O+(aq)
If MOH(1–q)+ is a strong base, the hydrolysis reaction does not occur. This is because the strong base completely dissociates. Thus, Group 1 and 2 metal ions (except Be2+) do not undergo significant hydrolysis.
For all other metal ions, the reaction is an equilibrium:
Mq+(aq) + 2 H2O(l) → ← MOH(q–1)+(aq) + H3O+(aq)
This reaction generates hydronium ion, so is also called acid hydrolysis.
Not surprisingly, the mass action expression is exactly like a Ka!
Ammonium ions also undergo cation hydrolysis. These are treated as the conjugate acid of the corresponding weak base.
Xp–(aq) + H2O(l) ? HX(p–1)–(aq) + OH–(aq)
If HX(p–1)– is a strong acid, then the hydrolysis reaction cannot occur because the strong acid completely dissociates. Thus, Cl–, Br–, I–, NO3–, ClO4–, and HSO4– do not undergo significant anion hydrolysis.
For other anions, this is an equilibrium reaction:
Xp–(aq) + H2O(l) → ← HX(p–1)–(aq) + OH–(aq)
Since hydroxide ion is generated, this reaction is also called base hydrolysis.
The mass action expression is identical to Kb!
Caution: anions with ionizable hydrogen atoms can undergo acid hydrolysis. To determine which reaction dominates, we need to compare the equilibrium constants: the larger equilibrium constant wins.
Determine whether aqueous solutions of the following salts are acidic, basic, or neutral.
1. Fe(NO3)2
2. NaF
3. ZnF2.