
In determining the nature of a Brønsted-Lowry acid-base reaction, it is found that the reaction always favors the weaker acid/base pair.
HNO3(aq) + H2O(l) → H3O+(aq) + NO3–(aq)
Strong Acid Weak Base Weak Conjugate Acid Weak Conjugate base
This means we need to be able to determine the relative strength of an acid or base by some means. Strong acids and bases are few and we have memorized these but when only weak species are involved, we need additional help.
HCN(aq) + H2O(l) → ← H3O+(aq) + CN–(aq)
Weak Acid Weak Base Weak Conjugate Acid Weak Conjugate base
Which direction is favored by the equilibrium?
Understanding the underlying principles of the structures of acids allows us to answer this question.
1) What are the charges on the species?
2) What is the atom-atom connectivity (structure)?
3) What are the electronegativities of atoms away from the acid hydrogen?
These principles are used to consider acid strength. We never consider base strength; rather, we look at the strength of the conjugate acid partner. Strong acids have weak conjugate base partners and weak acids have strong conjugate base partners.
Requirement for a binary acid: the second atom must be more electronegative than H.
H2O is an acid because H+ can be donated.
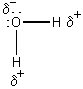
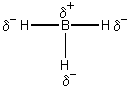
BH3 is not a Brønsted-Lowry acid because the H is negatively charged, thus cannot donate H+.
Using the three principles:
1) The most important contributor to acid strength is the overall charge of the species: the more negatively charged a species is, the poorer the acid strength. Why?
2) Structure: in binary acids the only structural feature is the X-H bond length:
As the bond length increases, the acid strength increases. Thus, larger X atoms lead to stronger acids.
3) As the electronegativity of X increases, the acid strength increases.
For binary acids, the balance between structure and electronegativity is close:
As atoms go down the Periodic Table, size is more important. The relative change in size is larger than the relative change in electronegativity as atoms go down the Periodic Table.
As atoms go across the Periodic Table, electronegativity changes are more important. The relative change in electronegativity is larger than the relative change in size as atoms go across the Periodic Table.
The consequence of this is, for binary acids:
Going down the Periodic Table increases acid strength.
Growing across to the right on the Periodic Table increases acid strength.
List the order of acid strength of the hydrogen halides.
Order the acid strength for CH4, NH3, H2O, HF.
These are the most common types of acids. Structurally, the acidic Hydrogen atoms are almost always bonded to an Oxygen atom. We can use Lewis dot structures to help us understand the reactivity:
(HO)p–X=Oq-p
Since the acid H is bonded to O, the OH bond length is always about the same in the compounds; this means that atomic size has no role in determining acid strength.
Charge and electronegativity are the only factors.
Charge is always the most important, just as in the binary acids.
The total electronegativity exerted upon the acidic hydrogen is a combination of the electronegativity of the central atom, X, and the oxo type oxygens. Since O has a high electronegativity, the number of oxygen atoms without H dominates. Consequently, the acid strength is primarily controlled by the number of oxo oxygens, p–q: a greater number of oxos leads to a greater acid strength. We only use the electronegativity of the central atom X if the number of oxo type oxygen atoms is the same.
Which is the stronger acid: H3PO4 or H3PO3?
What is the order of acidity for: H3AsO4, H2AsO4–, and HAsO42–?
Which is the strongest acid: H3PO4 or H2SO3?
Acid
: a substance that can accept an electron pair.Base
: a substance that can donate an electron pair.Acid/base reaction
: donation of an electron pair to create a new covalent bond.
This definition has no solvent or phase restrictions and is not dependent on any single chemical species. This is the most general acid/base definition we've looked at.
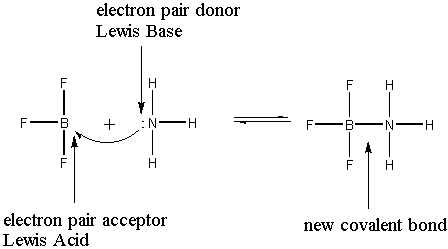
Identifying Lewis acid/base reactions usually means working with Lewis dot structures (not surprisingly, it's the same Lewis) so we can find electron pairs and new covalent bonds.
We will use Lewis acid/base concepts later in the course when we study complex ions:
Cu2+(aq) + 4 :NH3(aq) → ← [Cu(NH3)4]2+(aq)
Lewis Acid Lewis Base Complex Ion