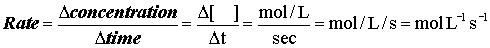Chemical Reactivity:
How fast do reactions proceed?
To what extent do reactions occur?
Can we predict the products of reactions?
What is the relationship between molecular structure and reactivity?
What are the energies associated with reactions?
To make the job more manageable we will limit ourselves to three types
of reactivity in aqueous solution:
Acid/Base
Precipitation
Oxidation/Reduction
Kinetics is the study of the quantitative aspects of the speed of reactions or rate at which a reaction occurs. Mathematically, this is expressed as: Factors influencing rates: Concentration of reactants Temperature Catalysts (materials that change the reaction rate without being consumed in the reaction) Surface area of a solid (either as a reactant or a catalyst) We will look at each of these aspects in our study of chemical kinetics.