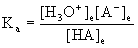Ka as a measure of acid strength.
Ka is defined for the reaction
HA(aq) + H2O(l) → ← H3O+(aq) + A–(aq)
With an equilibrium constant
A larger equilibrium constant means that the reaction has a greater tendency to drive towards the products side (more H3O+ and A–, less HA). Another way of saying this is that the acid has a stronger tendency to donate its H+, which is the definition of a stronger acid.