
HClO(aq) + H2O(l) → ← H3O+(aq) + ClO–(aq)
Ka = [H3O+]e[ClO–]e [HClO]e = 2.9×10–8
Initial0.010 0 0
Change–x +x +x
Equilibrium0.010 – x x x
Since Ka is pretty small, x will also be small (we are expecting it to be about 10–5 to 10–6). This means the chemistry has done a little bit of algebra for us:
0.010 – x ~ 0.010
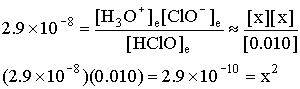
x = 1.7×10–5 = [H3O+]e
pH = –log[H3O+] = -log(1.7 ×10–5) = 4.77