
1. Write the balanced chemical reaction.
2 BrCl(g) → ← Br2(g) + Cl2(g)
2. Write the mass action expression.
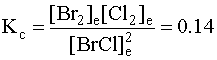
3. Set up a Table of Concentrations.
Initial 0.062 0 0
Change –2x +x +x
Equilibrium 0.062 – 2x x x
4. Substitute into the mass action expression:
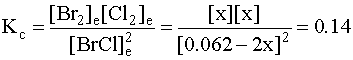
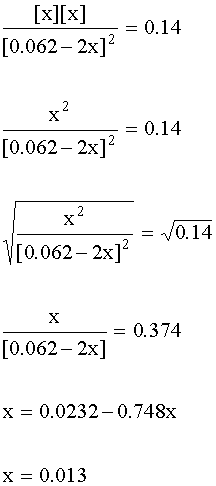
6. Substitute the variable into the equilibrium expressions.
[BrCl]e = 0.062 – 2x = 0.062 – 2(0.013) = 0.036 M
[Br2]e = [Cl2]e = x = 0.013 M
The answers can be checked by plugging them into the mass action expression and seeing if they match the equilibrium constant:
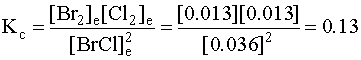
which is the same as the equilibrium constant, within experimental error.