1. Write the balanced chemical reaction.
2 BrCl(g)
→
←
Br2(g)
+
Cl2(g)
2. Write the mass action expression.
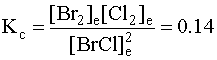
3. Set up a Table of Concentrations.
Initial
0.062
0
0
Change
–2x
+x
+x
Equilibrium
0.062 – 2x
x
x
4. Substitute into the mass action expression:
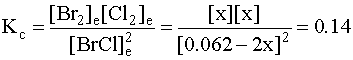
BackNext
