
HC2H3O2(aq) + H2O(l) → ← H3O+(aq) + C2H3O2–(aq)
Ka = [H3O+]e[C2H3O2–]e [HC2H3O2]e = 10–pKa = 10–4.77 = 1.7×10–5
Initial3.8×10–5 0 0
Change–x +x +x
Equilibrium3.8×10–5 – x x x
In this example, we expect x ~ 10–5, so we cannot make the approximation made earlier.
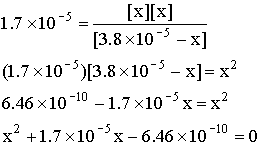
This must be solved using the quadratic equation to give:
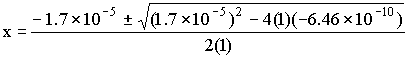
x = 1.8×10–5 or x = –3.5×10–5