

Consider the reaction
A(g) → products
Choose the data set corresponding the to eighth digit in your Student ID Number.
Find the activation energy in units of kJ/mol by plotting the data. Use a full, clean sheet of graph paper. (Spread sheets plots are NOT acceptable.) Be sure to label the axes and that the data encompasses the entire plotted area.
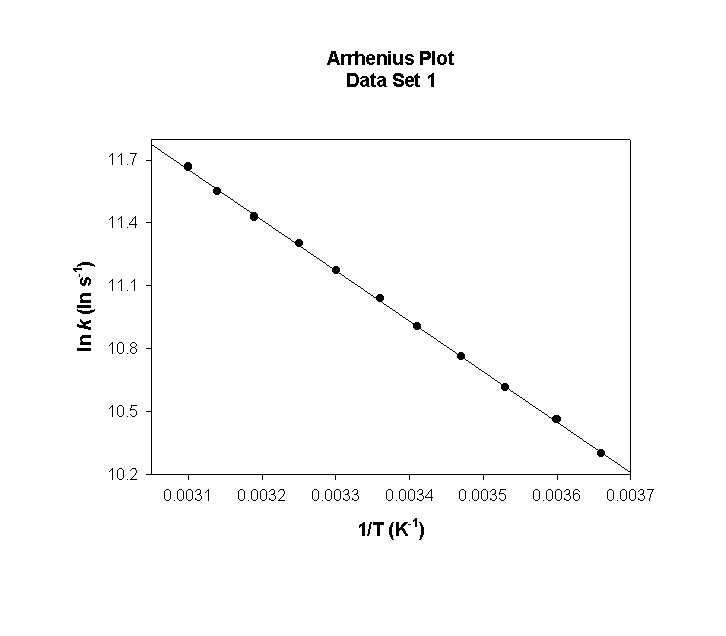
The Arrhenius equation can be written as
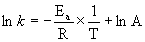
so a plot of ln k vs. 1/T (with T in units of K) gives the activation energy.
The shaded columns in the tables are the data plotted.
Then, Ea = –slope × R (R = 8.314 J/mol·K)
All answers are to 3 significant figures
Data Set 0
T (°C) T (K) 1/T (K–1) k (s–1) ln(k) (ln(s–1))
0 273 0.00366 0.14 –1.999
5 278 0.00360 0.20 –1.603
10 283 0.00353 0.30 –1.221
15 288 0.00347 0.43 –0.852
20 293 0.00341 0.61 –0.495
25 298 0.00336 0.86 –0.151
30 303 0.00330 1.2 0.182
35 308 0.00325 1.7 0.504
40 313 0.00319 2.3 0.816
45 318 0.00314 3.1 1.118
50 323 0.00310 4.1 1.411
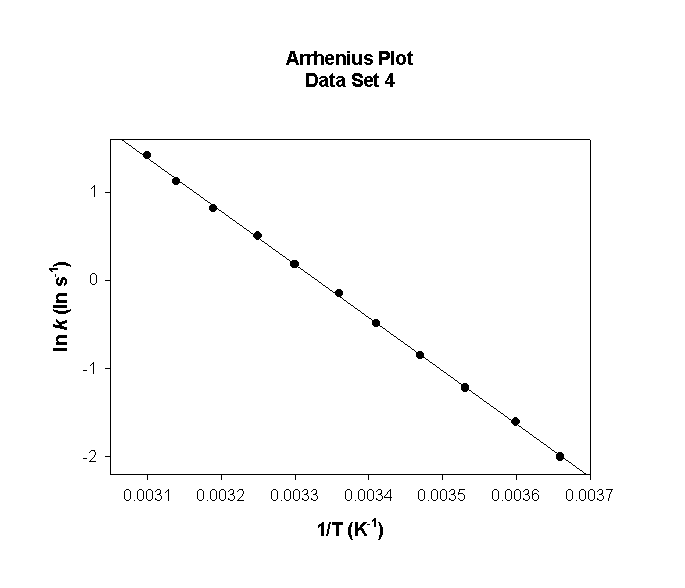
slope = –6030 so
Ea = –(–6030) × (8.314) = 50100 J/mol = 50.1 kJ/mol
Data Set 1
T (°C) T (K) 1/T (K–1) k (s–1) ln(k) (ln(s–1))
0 273 0.00366 1.22×106 14.015
5 278 0.00360 1.32×106 14.094
10 283 0.00353 1.43×106 14.171
15 288 0.00347 1.54×106 14.244
20 293 0.00341 1.65×106 14.316
25 298 0.00336 1.77×106 14.384
30 303 0.00330 1.89×106 14.451
35 308 0.00325 2.01×106 14.516
40 313 0.00319 2.14×106 14.578
45 318 0.00314 2.28×106 14.638
50 323 0.00310 2.41×106 14.697
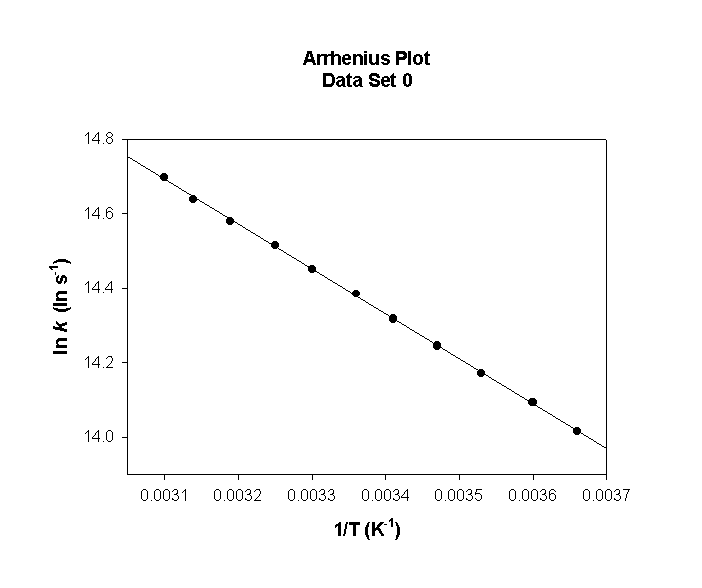
slope = –1210 so
Ea = –(–1210) × (8.314) = 10000 J/mol = 10.0 kJ/mol
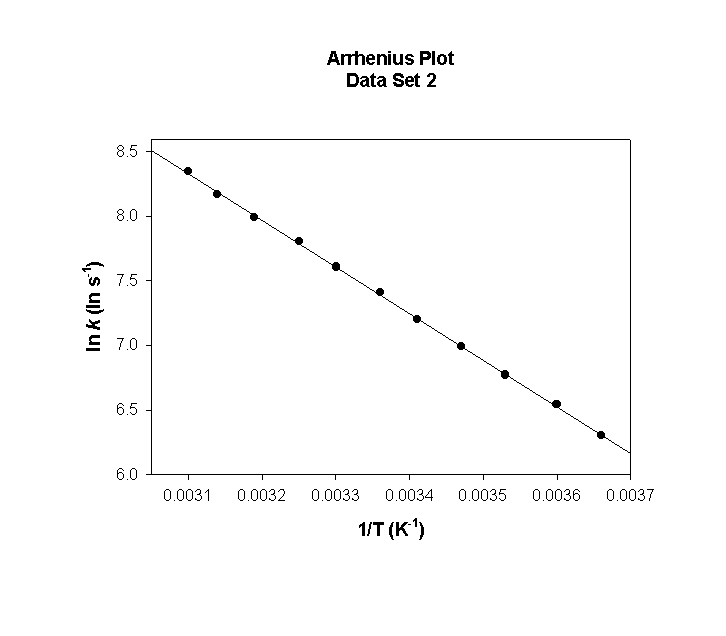
Data Set 2
T (°C) T (K) 1/T (K–1) k (s–1) ln(k) (ln(s–1))
0 273 0.00366 2.98×104 10.302
5 278 0.00360 3.49×104 10.461
10 283 0.00353 4.07×104 10.614
15 288 0.00347 4.72×104 10.761
20 293 0.00341 5.44×104 10.904
25 298 0.00336 6.24×104 11.041
30 303 0.00330 7.13×104 11.175
35 308 0.00325 8.11×104 11.304
40 313 0.00319 9.19×104 11.428
45 318 0.00314 1.04×105 11.549
50 323 0.00310 1.17×105 11.666
slope = –2410 so
Ea = –(–2410) × (8.314) = 20000 J/mol = 20.0 kJ/mol
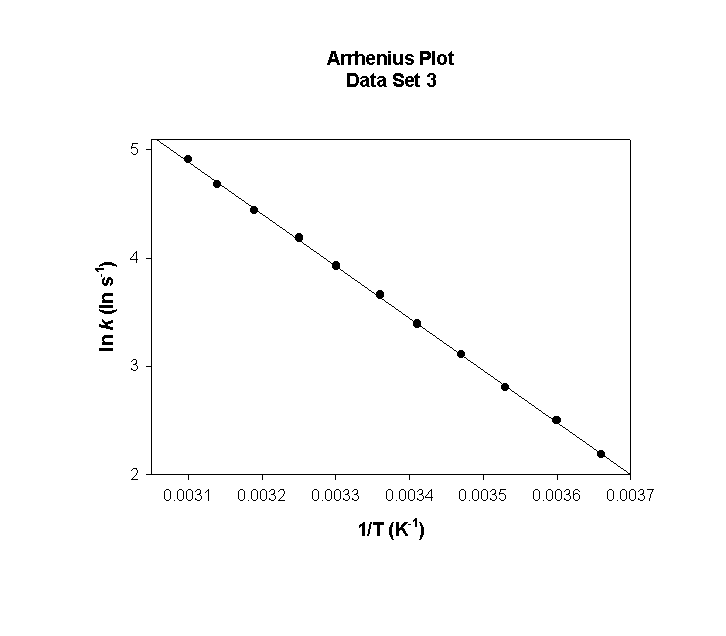
Data Set 3
T (°C) T (K) 1/T (K–1) k (s–1) ln(k) (ln(s–1))
0 273 0.00366 7.34×10–11 –23.335
5 278 0.00360 1.62×10–10 –22.543
10 283 0.00353 3.48×10–10 –21.778
15 288 0.00347 7.28×10–10 –21.040
20 293 0.00341 1.49×10–9 –20.328
25 298 0.00336 2.96×10–9 –19.639
30 303 0.00330 5.76×10–9 –18.973
35 308 0.00325 1.10×10–8 –18.328
40 313 0.00319 2.05×10–8 –17.705
45 318 0.00314 3.74×10–8 –17.100
50 323 0.00310 6.73×10–8 –16.515
slope = –12100 so
Ea = –(–12100) × (8.314) = 100000 J/mol = 100. kJ/mol
Data Set 4
T (°C) T (K) 1/T (K–1) k (s–1) ln(k) (ln(s–1))
0 273 0.00366 5.41×10–9 –19.035
5 278 0.00360 1.10×10–8 –18.321
10 283 0.00353 2.20×10–8 –17.633
15 288 0.00347 4.27×10–8 –16.969
20 293 0.00341 8.11×10–8 –16.328
25 298 0.00336 1.51×10–7 –15.708
30 303 0.00330 2.74×10–7 –15.109
35 308 0.00325 4.90×10–7 –14.529
40 313 0.00319 8.59×10–7 –13.967
45 318 0.00314 1.48×10–6 –13.423
50 323 0.00310 2.51×10–6 –12.896
slope = –10800 so
Ea = –(–10800) × (8.314) = 89800 J/mol = 89.8 kJ/mol
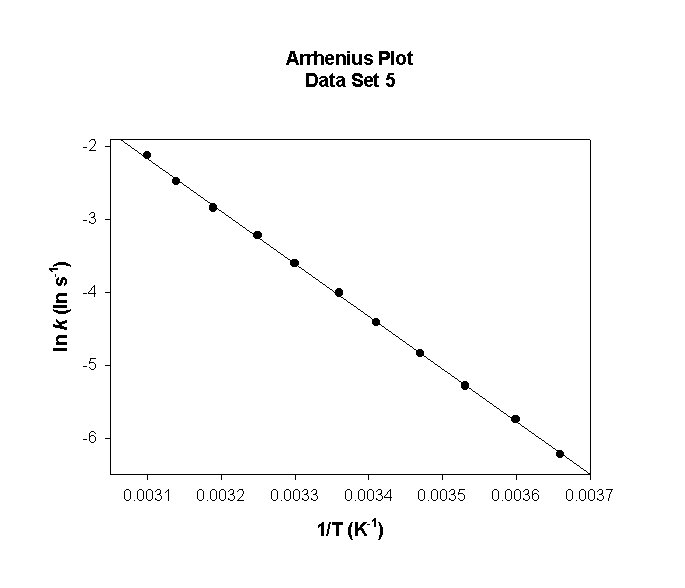
Data Set 5
T (°C) T (K) 1/T (K–1) k (s–1) ln(k) (ln(s–1))
0 273 0.00366 8.88 2.184
5 278 0.00360 12.2 2.501
10 283 0.00353 16.6 2.806
15 288 0.00347 22.2 3.102
20 293 0.00341 29.6 3.387
25 298 0.00336 38.9 3.662
30 303 0.00330 50.8 3.929
35 308 0.00325 65.8 4.186
40 313 0.00319 84.4 4.436
45 318 0.00314 107.5 4.678
50 323 0.00310 135.9 4.912
slope = –4820 so
Ea = –(–4820) × (8.314) = 40100 J/mol = 40.1 kJ/mol
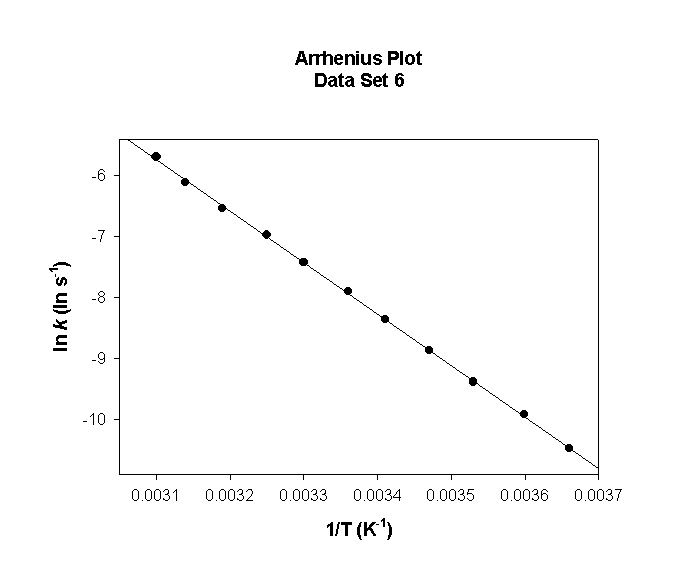
Data Set 6
T (°C) T (K) 1/T (K–1) k (s–1) ln(k) (ln(s–1))
0 273 0.00366 3.94×10–7 –14.746
5 278 0.00360 7.43×10–7 –14.113
10 283 0.00353 1.37×10–6 –13.501
15 288 0.00347 2.47×10–6 –12.911
20 293 0.00341 4.37×10–6 –12.341
25 298 0.00336 7.58×10–6 –11.790
30 303 0.00330 1.29×10–5 –11.257
35 308 0.00325 2.16×10–5 –10.741
40 313 0.00319 3.56×10–5 –10.242
45 318 0.00314 5.78×10–5 –9.759
50 323 0.00310 9.23×10–5 –9.290
slope = –9640 so
Ea = –(–9640) × (8.314) = 80100 J/mol = 80.1 kJ/mol
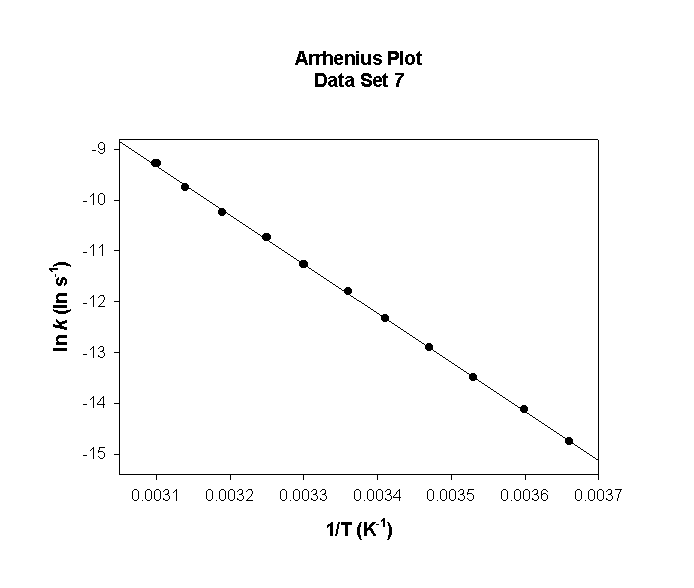
Data Set 7
T (°C) T (K) 1/T (K–1) k (s–1) ln(k) (ln(s–1))
0 273 0.00366 2.83×10–5 –10.474
5 278 0.00360 4.92×10–5 –9.920
10 283 0.00353 8.40×10–5 –9.384
15 288 0.00347 1.41×10–4 –8.868
20 293 0.00341 2.32×10–4 –8.369
25 298 0.00336 3.76×10–4 –7.887
30 303 0.00330 5.99×10–4 –7.421
35 308 0.00325 9.40×10–4 –6.970
40 313 0.00319 1.45×10–3 –6.533
45 318 0.00314 2.22×10–3 –6.110
50 323 0.00310 3.35×10–3 –5.700
slope = –8440 so
Ea = –(–8440) × (8.314) = 70200 J/mol = 70.2 kJ/mol
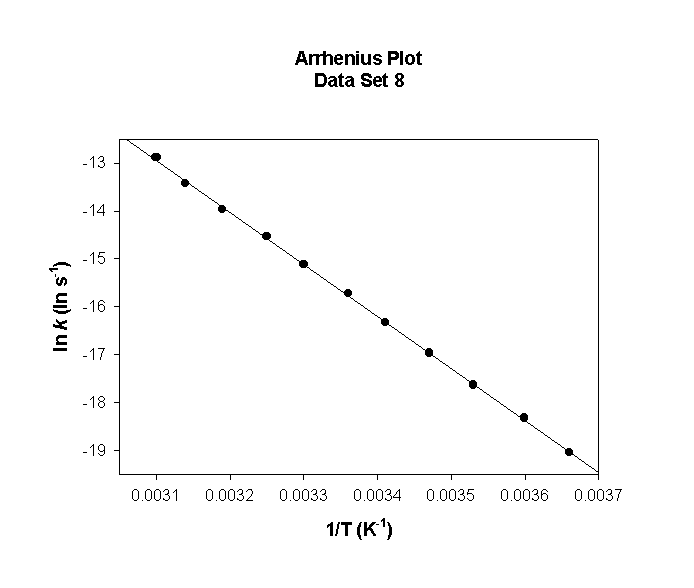
Data Set 8
T (°C) T (K) 1/T (K–1) k (s–1) ln(k) (ln(s–1))
0 273 0.00366 1.98×10–3 –6.223
5 278 0.00360 3.19×10–3 –5.747
10 283 0.00353 5.05×10–3 –5.288
15 288 0.00347 7.86×10–3 –4.846
20 293 0.00341 1.21×10–2 –4.418
25 298 0.00336 1.82×10–2 –4.005
30 303 0.00330 2.72×10–2 –3.605
35 308 0.00325 4.00×10–2 –3.219
40 313 0.00319 5.82×10–2 –2.844
45 318 0.00314 8.36×10–2 –2.482
50 323 0.00310 1.19×10–1 –2.130
slope = –7230 so
Ea = –(–7230) × (8.314) = 60100 J/mol = 60.1 kJ/mol
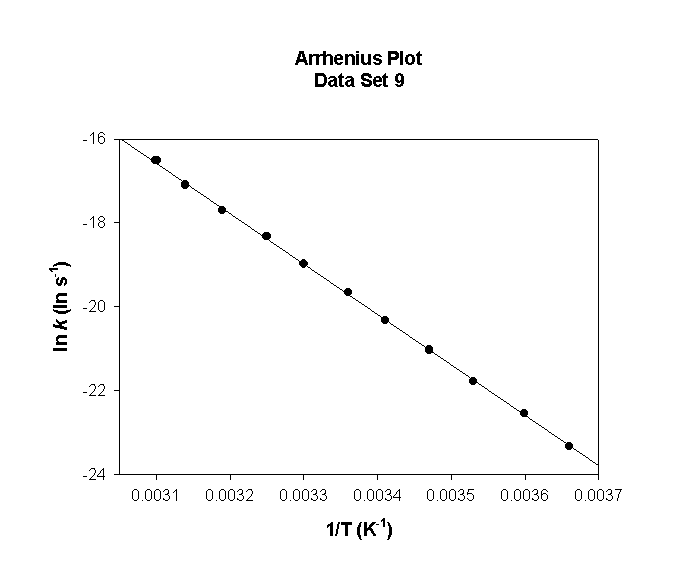
Data Set 9
T (°C) T (K) 1/T (K–1) k (s–1) ln(k) (ln(s–1))
0 273 0.00366 5.46×102 6.302
5 278 0.00360 6.92×102 6.540
10 283 0.00353 8.70×102 6.769
15 288 0.00347 1.09×103 6.990
20 293 0.00341 1.34×103 7.204
25 298 0.00336 1.65×103 7.411
30 303 0.00330 2.02×103 7.610
35 308 0.00325 2.45×103 7.804
40 313 0.00319 2.95×103 7.991
45 318 0.00314 3.54×103 8.172
50 323 0.00310 4.22×103 8.348
slope = –3620 so
Ea = –(–3620) × (8.314) = 30100 J/mol = 30.1 kJ/mol